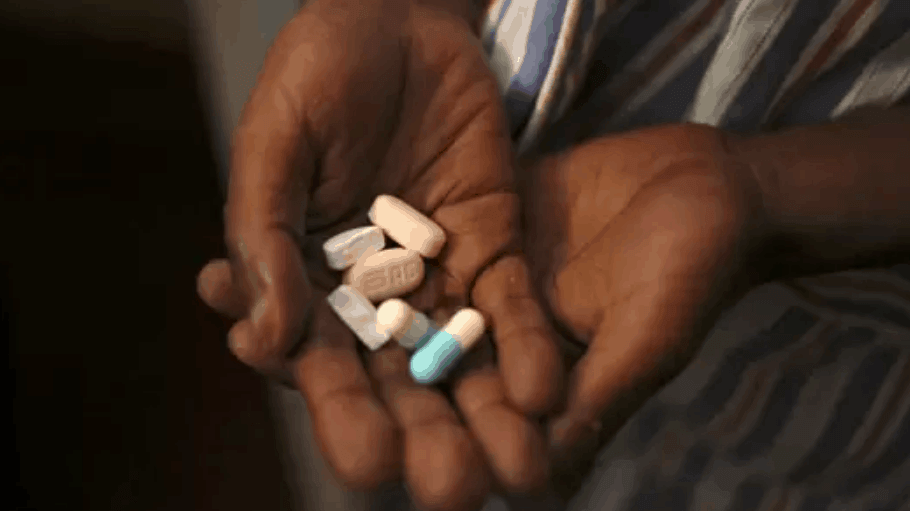
By Stavros Nicolau, published in Independent Media’s Business Report JOHANNESBURG – Last week’s launch by Minister of Health Zweli Mkhize of a new ARV triple drug regimen in the Ugu district in KwaZulu-Natal and the commemoration of World Aids Day allows us as a country to reflect on how successfully we’ve managed what is arguably… Continue reading OPINION: Let’s pause for a moment to reflect on SA’s ARV success
OPINION: Let’s pause for a moment to reflect on SA’s ARV success